12024届全国名校高三单元检测示范卷(二十一)化学试卷答案试卷答案,我们目前收集并整理关于12024届全国名校高三单元检测示范卷(二十一)化学试卷答案得系列试题及其答案,更多试题答案请关注本网站

12024届全国名校高三单元检测示范卷(二十一)化学试卷答案试卷答案
以下是该试卷的部分内容或者是答案亦或者啥也没有
moreboad.Inovationcanhelptradrsgofur-ceandsomeofthemevencometwicetoexperienceewPa民”hedsireddinnovationoftraditionaloperaimportant?Succe黑afterfashion.Tattracttheyoungaudiences.Toimprovedigitalstageeffects.DTochangethetraditionaloperacircle.hichofthefollowingcanbestdescribeTianleyuanTheater?AAdestroyerofPekingOpera.Apioneerofinnovativeopera.CAnobjectorofmodernopera.第DAdefenderoftraditionalopera.第doesDengfeelabouttheinnovationofjingju?AHelpful.B.Unreliable.C.Worthless.D.Conservative.hatisthemainideaofthetext?onThetraditionaloperaisdyingout.Thefutureofthetraditionaloperaispromising.Traditionaloperacircleisinnovatingtoattractyoungaudiencesshformersofthetraditionaloperaaresupportingjingju.of《典5小题:每小题2.5分,满分12.5分)面文,从短文后的选项中选出可以填入空白处的最佳选项
选项中有两项为多余sa海球tryingtofigureoutexactlyhowoftentheiremployeeswillneedhasbecomeclear.Researchshowsthatmanyworkerswantflexi-trilinadnesgns,but斜toyourcompanyandincorporateitintoyourrequest.Soifyouduringwaboutproductivity,saysomethinglike:OverthepastyearI'veere'salsoeworkinglikethis.37.sAea8eF网stangetradBlackFBebilitytoyoumightmeanworkingremotethreedaysaweek,buttoPeopleiyourbworkingfromhomeonceaweek.3.YouwanttobespecifielsonBlawithwhattheneedisandthewayyouwouldliketohavemoreflexibility.Suggestingaanksgivinperiodoftheproposedschedule,likehavingareviewinthreemonths,canmakeammorelikelytoagree.BlackFMaketherequestinpersonwhichcoIfpossible,havethisconversationinpersonorovervideoconferenceRthatpeoplearemorelikelytogetwhattheywantwhentheyasknnotosomeone'sface.It'sveryuncomfortable.39Theyh【高三英语第7页(共1费)1
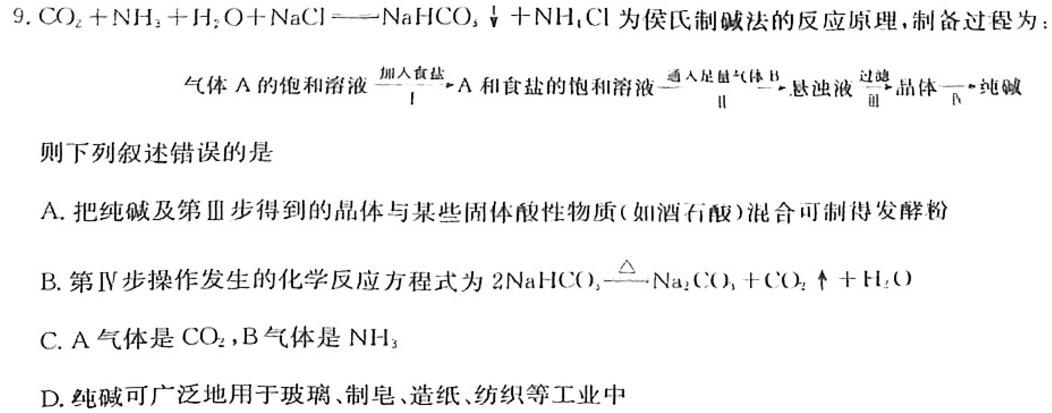
9.CO2+NH3+H2O+NaClNaHCO,N+NH4Cl为侯氏制碱法的反应原理,制备过程为:体A的饱和溶液A和食盐的饱和溶液一人足气体BB.悬浊液品体纯则下列叙述错误的是B.第Ⅳ步操作发生的化学反应方程式为A.把纯碱及第Ⅲ步得到的晶体与某些固体酸性物质(如酒石酸)混合可制得发酵粉2NaHCO3Na2CO3+CO2+H2OC.A气体是CO2,B气体是NH3D.纯碱可广泛地用于玻璃、制皂、造纸、纺织等工业中

2.8molH2(g)(2)一定条件下,向2L恒温恒容密闭容器中充入1molCO2(g)和仅发生反应i,在两种不同催化剂作用下建立平衡的过程(过程I、过程Ⅱ)中,CO2的转化率[(CO2)]随反应时间(t)的变化曲线如图1所示。①a点v正b点v(填“>”“<”或“=”)。②已知平衡时a点总压强为,则该反应的压强平衡常数p,KP=。③过程I,t2时刻改变的反应条件可能是(填两条)。(3)CO2和H2反应合成甲醇的过程中,还会有以下副反应发生: